Our Lead Programs

We are currently advancing a pipeline of novel NAD-boosting compounds:

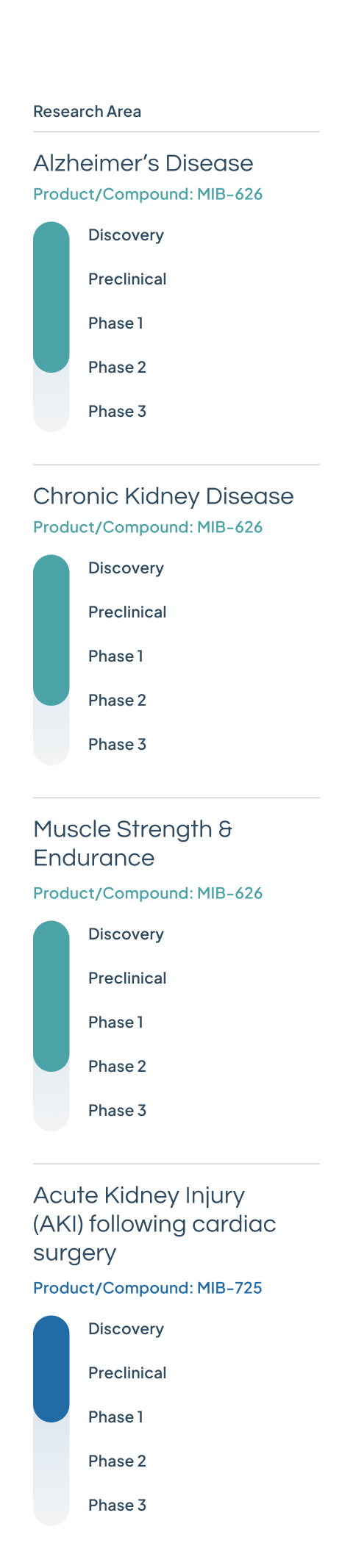
MIB-626
Our lead drug candidate, MIB-626, is a novel, patented crystalline NAD+ booster with scalable manufacturing capabilities. It is currently in three Phase 2 clinical trials for Alzheimer’s disease, chronic kidney disease, and muscle strength and endurance.

MIB-725
A next-generation NAD+ precursor that will be under investigation for acute kidney injury (AKI) following cardiac surgery.
Phase 1 safety and bioavailability studies commenced in Q1 2025.
In addition, we are progressing clinical development in multiple related areas:
01 Muscle Strength Trials
Enrollment concluded for a key study with results anticipated later this year.
02 Alzheimer’s Disease
A Phase 2a trial is underway, with recruitment completed and preliminary data from the study being available possible by year-end. Ongoing studies are addressing mild cognitive impairment and early dementia.
03 Diabetic Kidney Disease
Our DKD program has expanded to three clinical sites, including a new location in Los Angeles; primary readout expected in 2026.